Should the cleaning course of action continuously cuts down the contaminants to some degree in the limit of acceptance standards, then the process remaining followed for cleaning might be thought to be validated.
The product chosen from a gaggle of products which represents the greatest possibility of carry-in excess of contamination to other items designed in exactly the same devices by advantage of its weak solubility, potency, and toxicity, or a combination of these components.
If no cleaning validation required or not accomplished on the following worst-case inside 03 several years then revalidation shall be completed on current worst within the frequency of 03 a long time,
Be aware: If the cleaning method is staying changed following the failure of the result nevertheless three consecutive cleaning runs really should be validated using a improved cleaning method.
Not more than 10ppm of your prior products really should look in a very subsequently created succeeding products.
I can revoke my consent at any time with influence for the long run by sending an e-mail to unsubscribe@sartorius.com or by clicking on the "unsubscribe" hyperlink in e-mails I've acquired.
Right away following wetting website the swab wick, swab the specified machines surfaces as per the sampling approach.
Generation/QA personal evaluating Visible cleanliness shall be properly trained for observing and figuring out drug substances at very low-amount focus.
If any solvents are utilized for cleaning of equipment, success obtained for residual solvent should be under 1/tenth on the ICH specified Restrict. The identical shall be noted from the respective cleaning validation report.
Swab sampling web page shall not be recurring and re-swabbing shall not be performed through the same location of apparatus wherever the swab sample is by now collected in advance of.
In the case wherever the solubility profile of two or even more products and solutions is identical, the merchandise acquiring the highest toughness shall be chosen as being the worst circumstance in this criterion.
Immersion Method: The immersion method may be possibly agitated, where by a cleaning agent inside of a system vessel is mechanically stimulated, or static, the place the method vessel is soaked While using the cleaning agent.
A scientific method, cleaning validation is seal of authentication for the cleaning technique's efficiency. It website involves the elimination of dirt, germs, bacteria & microbes from surfaces & environments.
Transfer the swab applying gloved worn hand into the examination tube and review it According to the validated analytical method.
 Neve Campbell Then & Now!
Neve Campbell Then & Now!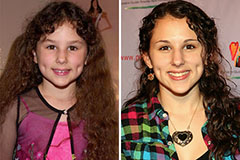 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Michael Fishman Then & Now!
Michael Fishman Then & Now! Mason Reese Then & Now!
Mason Reese Then & Now! Stephen Hawking Then & Now!
Stephen Hawking Then & Now!